Ionic bond
(materials from the site http://www.hemi.nsu.ru/ucheb138.htm were used)
Ionic bonding occurs through electrostatic attraction between oppositely charged ions. These ions are formed as a result of the transfer of electrons from one atom to another. An ionic bond is formed between atoms that have large differences in electronegativity (usually greater than 1.7 on the Pauling scale), for example, between alkali metal and halogen atoms.
Let us consider the occurrence of an ionic bond using the example of the formation of NaCl.
From electronic formulas of atoms
Na 1s 2 2s 2 2p 6 3s 1 and
Cl 1s 2 2s 2 2p 6 3s 2 3p 5
It can be seen that to complete the outer level, it is easier for a sodium atom to give up one electron than to gain seven, and for a chlorine atom it is easier to gain one electron than to gain seven. In chemical reactions, the sodium atom gives up one electron, and the chlorine atom takes it. As a result, the electron shells of sodium and chlorine atoms are transformed into stable electron shells of noble gases (electronic configuration of the sodium cation
Na + 1s 2 2s 2 2p 6,
and the electronic configuration of the chlorine anion is
Cl – - 1s 2 2s 2 2p 6 3s 2 3p 6).
The electrostatic interaction of ions leads to the formation of a NaCl molecule.
The nature of the chemical bond is often reflected in the state of aggregation and physical properties of the substance. Ionic compounds such as sodium chloride NaCl are hard and refractory because there are powerful forces of electrostatic attraction between the charges of their “+” and “–” ions.
The negatively charged chlorine ion attracts not only “its” Na+ ion, but also other sodium ions around it. This leads to the fact that near any of the ions there is not one ion with the opposite sign, but several.
The structure of a crystal of sodium chloride NaCl.
In fact, there are 6 sodium ions around each chlorine ion, and 6 chlorine ions around each sodium ion. This ordered packing of ions is called an ionic crystal. If a single chlorine atom is isolated in a crystal, then among the sodium atoms surrounding it it is no longer possible to find the one with which the chlorine reacted.
Attracted to each other by electrostatic forces, the ions are extremely reluctant to change their location under the influence of external force or an increase in temperature. But if sodium chloride is melted and continued to be heated in a vacuum, it evaporates, forming diatomic NaCl molecules. This suggests that covalent bonding forces are never completely turned off.
Basic characteristics of ionic bonds and properties of ionic compounds
1. An ionic bond is a strong chemical bond. The energy of this bond is on the order of 300 – 700 kJ/mol.
2. Unlike a covalent bond, an ionic bond is non-directional because an ion can attract ions of the opposite sign to itself in any direction.
3. Unlike a covalent bond, an ionic bond is unsaturated, since the interaction of ions of the opposite sign does not lead to complete mutual compensation of their force fields.
4. During the formation of molecules with an ionic bond, complete transfer of electrons does not occur, therefore, one hundred percent ionic bonds do not exist in nature. In the NaCl molecule, the chemical bond is only 80% ionic.
5. Compounds with ionic bonds are crystalline solids that have high melting and boiling points.
6. Most ionic compounds are soluble in water. Solutions and melts of ionic compounds conduct electric current.
Metal connection
Metal crystals are structured differently. If you examine a piece of sodium metal, you will find that its appearance is very different from table salt. Sodium is a soft metal, easily cut with a knife, flattened with a hammer, it can be easily melted in a cup on an alcohol lamp (melting point 97.8 o C). In a sodium crystal, each atom is surrounded by eight other similar atoms.

Crystal structure of metallic Na.
The figure shows that the Na atom in the center of the cube has 8 nearest neighbors. But the same can be said about any other atom in a crystal, since they are all the same. The crystal consists of "infinitely" repeating fragments shown in this figure.
Metal atoms at the outer energy level contain a small number of valence electrons. Since the ionization energy of metal atoms is low, valence electrons are weakly retained in these atoms. As a result, positively charged ions and free electrons appear in the crystal lattice of metals. In this case, metal cations are located in the nodes of the crystal lattice, and electrons move freely in the field of positive centers, forming the so-called “electron gas”.
The presence of a negatively charged electron between two cations causes each cation to interact with this electron.
Thus, Metallic bonding is the bonding between positive ions in metal crystals that occurs through the attraction of electrons moving freely throughout the crystal.
Since the valence electrons in a metal are evenly distributed throughout the crystal, a metallic bond, like an ionic bond, is a non-directional bond. Unlike a covalent bond, a metallic bond is an unsaturated bond. A metal bond also differs from a covalent bond in strength. The energy of a metallic bond is approximately three to four times less than the energy of a covalent bond.
Due to the high mobility of the electron gas, metals are characterized by high electrical and thermal conductivity.
The metal crystal looks quite simple, but in fact its electronic structure is more complex than that of ionic salt crystals. There are not enough electrons in the outer electron shell of metal elements to form a full-fledged “octet” covalent or ionic bond. Therefore, in the gaseous state, most metals consist of monatomic molecules (i.e., individual atoms not connected to each other). A typical example is mercury vapor. Thus, the metallic bond between metal atoms occurs only in the liquid and solid state of aggregation.
A metallic bond can be described as follows: some of the metal atoms in the resulting crystal give up their valence electrons to the space between the atoms (for sodium this is...3s1), turning into ions. Since all the metal atoms in a crystal are the same, each has an equal chance of losing a valence electron.
In other words, the transfer of electrons between neutral and ionized metal atoms occurs without energy consumption. In this case, some electrons always end up in the space between the atoms in the form of “electron gas”.
These free electrons, firstly, hold the metal atoms at a certain equilibrium distance from each other.
Secondly, they give metals a characteristic “metallic shine” (free electrons can interact with light quanta).
Thirdly, free electrons provide metals with good electrical conductivity. The high thermal conductivity of metals is also explained by the presence of free electrons in the interatomic space - they easily “respond” to changes in energy and contribute to its rapid transfer in the crystal.

A simplified model of the electronic structure of a metal crystal.
******** Using the metal sodium as an example, let us consider the nature of the metallic bond from the point of view of ideas about atomic orbitals. The sodium atom, like many other metals, has a lack of valence electrons, but there are free valence orbitals. The only 3s electron of sodium is capable of moving to any of the free and close-in-energy neighboring orbitals. As atoms in a crystal come closer together, the outer orbitals of neighboring atoms overlap, allowing the electrons given up to move freely throughout the crystal.
However, the "electron gas" is not as disorderly as it might seem. Free electrons in a metal crystal are in overlapping orbitals and are to some extent shared, forming something like covalent bonds. Sodium, potassium, rubidium and other metallic s-elements simply have few shared electrons, so their crystals are fragile and fusible. As the number of valence electrons increases, the strength of metals generally increases.
Thus, metallic bonds tend to be formed by elements whose atoms have few valence electrons in their outer shells. These valence electrons, which carry out the metallic bond, are shared so much that they can move throughout the metal crystal and provide high electrical conductivity of the metal.
A NaCl crystal does not conduct electricity because there are no free electrons in the space between the ions. All electrons donated by sodium atoms are firmly held by chlorine ions. This is one of the significant differences between ionic crystals and metal ones.
What you now know about metallic bonding helps explain the high malleability (ductility) of most metals. Metal can be flattened into a thin sheet and drawn into wire. The fact is that individual layers of atoms in a metal crystal can slide one another relatively easily: the mobile “electron gas” constantly softens the movement of individual positive ions, shielding them from each other.
Of course, nothing like this can be done with table salt, although salt is also a crystalline substance. In ionic crystals, the valence electrons are tightly bound to the nucleus of the atom. The shift of one layer of ions relative to another brings ions of the same charge closer together and causes strong repulsion between them, resulting in the destruction of the crystal (NaCl is a fragile substance).

The shift of the layers of an ionic crystal causes the appearance of large repulsive forces between like ions and destruction of the crystal.
Navigation
- Solving combined problems based on quantitative characteristics of a substance
- Problem solving. The law of constancy of the composition of substances. Calculations using the concepts of “molar mass” and “chemical amount” of a substance
The purpose of the lesson
- Give an idea of metal chemical bonding.
- Learn to write down patterns of metal bond formation.
- Get acquainted with the physical properties of metals.
- Learn to clearly distinguish between species chemical bonds .
Lesson Objectives
- Find out how they interact with each other metal atoms
- Determine how a metal bond affects the properties of the substances formed by it
Key terms:
- Electronegativity - a chemical property of an atom, which is a quantitative characteristic of the ability of an atom in a molecule to attract common electron pairs.
- Chemical bond -the phenomenon of interaction of atoms, due to the overlap of electron clouds of interacting atoms.
- Metal connection is a bond in metals between atoms and ions, formed through the sharing of electrons.
- Covalent bond - a chemical bond formed by overlapping a pair of valence electrons. The electrons that provide the connection are called a common electron pair. There are 2 types: polar and non-polar.
- Ionic bond - a chemical bond that forms between non-metal atoms, in which a shared electron pair goes to an atom with higher electronegativity. As a result, atoms attract like oppositely charged bodies.
- Hydrogen bond
- a chemical bond between an electronegative atom and a hydrogen atom H bonded covalently to another electronegative atom. Electronegative atoms can be N, O or F. Hydrogen bonds can be intermolecular or intramolecular.
DURING THE CLASSES
Metal chemical bond
Identify the elements that are in the wrong “queue”. Why?
Ca Fe P K Al Mg Na
Which elements from the table Mendeleev are called metals?
Today we will learn what properties metals have, and how they depend on the bond that is formed between the metal ions.
First, let's remember the location of metals in the periodic table?
Metals, as we all know, usually do not exist in the form of isolated atoms, but in the form of a piece, ingot or metal product. Let's find out what collects metal atoms in a complete volume.
In the example we see a piece of gold. And by the way, gold is a unique metal. Using forging, pure gold can be used to make foil 0.002 mm thick! This thin sheet of foil is almost transparent and has a green tint in the light. As a result, from an ingot of gold the size of a matchbox, you can get a thin foil that will cover the area of a tennis court.
Chemically, all metals are characterized by the ease of giving up valence electrons, and as a result, the formation of positively charged ions and exhibit only positive oxidation. That is why metals in a free state are reducing agents. A common feature of metal atoms is their large size relative to non-metals. The outer electrons are located at large distances from the nucleus and are therefore weakly connected to it, therefore they are easily separated.
Atoms of a larger number of metals at the external level have a small number of electrons - 1,2,3. These electrons are easily stripped off and the metal atoms become ions.
Ме0 – n ē ⇆ Men+
metal atoms – electrons ext. orbits ⇆ metal ions
In this way, the detached electrons can move from one ion to another, that is, they become free, as if linking them into a single whole. Therefore, it turns out that all the detached electrons are common, since it is impossible to understand which electron belongs to which of the metal atoms.
Electrons can combine with cations, then atoms are temporarily formed, from which electrons are then torn off. This process occurs constantly and without stopping. It turns out that in the volume of the metal, atoms are continuously transformed into ions and vice versa. In this case, a small number of shared electrons binds a large number of metal atoms and ions. But it is important that the number of electrons in the metal is equal to the total charge of the positive ions, that is, it turns out that in general the metal remains electrically neutral.
This process is presented as a model - metal ions are in a cloud of electrons. Such an electron cloud is called an “electron gas.”
For example, in this picture we see how electrons move among motionless ions inside the crystal lattice of metal. 
Rice. 2. Electron movement
In order to better understand what Electron gas is and how it behaves in chemical reactions of different metals, let’s watch an interesting video. (gold is only mentioned as a color in this video!)
Now we can write down the definition: a metallic bond is a bond in metals between atoms and ions, formed by sharing electrons.
Let's compare all the types of connections that we know and consolidate them in order to better distinguish them, for this we will watch the video.
Metallic bonding occurs not only in pure metals, but is also characteristic of mixtures of different metals and alloys in different states of aggregation.
The metallic bond is important and determines the basic properties of metals
- electrical conductivity – random movement of electrons in the volume of metal. But with a small potential difference, so that the electrons move in an orderly manner. Metals with the best conductivity are Ag, Cu, Au, Al.
- plasticity
The bonds between the metal layers are not very significant, this allows the layers to move under load (deform the metal without breaking it). The best deformable metals (soft) are Au, Ag, Cu.
- metallic shine
Electron gas reflects almost all light rays. This is why pure metals shine so much and most often have a gray or white color. Metals that are the best reflectors Ag, Cu, Al, Pd, Hg
Homework
Exercise 1
Choose the formulas of substances that have
a) covalent polar bond: Cl2, KCl, NH3, O2, MgO, CCl4, SO2;
b) with ionic bond: HCl, KBr, P4, H2S, Na2O, CO2, CaS.
Exercise 2
Cross out the extra:
a) CuCl2, Al, MgS
b) N2, HCl, O2
c) Ca, CO2, Fe
d) MgCl2, NH3, H2
Sodium metal, lithium metal, and other alkali metals change the color of the flame. Metallic lithium and its salts give the fire a red color, metallic sodium and sodium salts give it a yellow color, metallic potassium and its salts give it a purple color, and rubidium and cesium give it a purple color, but lighter.
Rice. 4. A piece of lithium metal 
Rice. 5. Flame coloring with metals
Lithium (Li). Lithium metal, like sodium metal, is an alkali metal. Both are soluble in water. Sodium, when dissolved in water, forms caustic soda, a very strong acid. When alkali metals are dissolved in water, a lot of heat and gas (hydrogen) are released. It is advisable not to touch such metals with your hands, as you may get burned.
Bibliography
1. Lesson on the topic “Metallic chemical bond”, chemistry teacher Tukhta Valentina Anatolyevna MOU "Yesenovichskaya Secondary School"
2. F. A. Derkach “Chemistry” - scientific and methodological manual. – Kyiv, 2008.
3. L. B. Tsvetkova “Inorganic chemistry” - 2nd edition, corrected and expanded. – Lvov, 2006.
4. V. V. Malinovsky, P. G. Nagorny “Inorganic chemistry” - Kyiv, 2009.
5. Glinka N.L. General chemistry. – 27th ed./Under. ed. V.A. Rabinovich. – L.: Chemistry, 2008. – 704 pp.
Edited and sent by Lisnyak A.V.
Worked on the lesson:
Tukhta V.A.
Lisnyak A.V.
You can raise a question about modern education, express an idea or solve a pressing problem at Educational forum, where an educational council of fresh thought and action meets internationally. Having created blog, Chemistry 8th grade
Metal connection
As a result of electrostatic attraction between the cation and anion, a molecule is formed.
Ionic bond
The theory of ionic bonding was proposed by 1916 ᴦ. German scientist W. Kossel. This theory explains the formation of connections between atoms of typical metals and atoms typical non-metals: CsF, CsCl, NaCl, KF, KCl, Na 2 O, etc.
According to this theory, when an ionic bond is formed, atoms of typical metals give up electrons, and atoms of typical nonmetals accept electrons.
As a result of these processes, metal atoms are transformed into positively charged particles, which are called positive ions or cations; and non-metal atoms turn into negative ions - anions. The charge of the cation is equal to the number of electrons given up.
Metal atoms donate electrons to their outer layer, and the resulting ions have complete electronic structures (pre-outer electronic layer).
The magnitude of the negative charge of the anion is equal to the number of electrons accepted.
Non-metal atoms accept the number of electrons that is extremely important for them to completion of an electronic octet (outer electronic layer).
For example: the general scheme for the formation of a NaCl molecule from Na and C1 atoms: Na°-le = Na +1 Formation of ions
Сl°+1е - = Сl -
Na +1 + Cl - = Na + Cl -
Na°+ Сl°= Na + Сl - Compound of ions
· The bond between ions is commonly called ionic bonding.
Compounds that consist of ions are called ionic compounds.
The algebraic sum of the charges of all ions in the molecule of an ionic compound must be equal to zero, because any molecule is an electrically neutral particle.
There is no sharp boundary between ionic and covalent bonds. An ionic bond can be considered as an extreme case of a polar covalent bond, in which the formation of a shared electron pair completely moves towards the atom with higher electronegativity.
Most typical metal atoms have a small number of electrons in their outer electron layer (typically 1 to 3); these electrons are called valence electrons. In metal atoms, the strength of the bond between the valence electrons and the nucleus is low, that is, the atoms have low ionization energy. This makes it easy to lose valence electrons h transformation of metal atoms into positively charged ions (cations):
Ме° -ne ® Ме n +
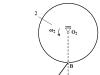
In the crystal structure of a metal, valence electrons have the ability to easily move from one atom to another, which leads to the sharing of electrons by all neighboring atoms. In a simplified way, the structure of a metal crystal is represented as follows: at the nodes of the crystal lattice there are Me n+ ions and Me° atoms, and valence electrons move relatively freely between them, establishing connections between all atoms and ions of the metal (Fig. 3). This is a special type of chemical bond called a metal bond.
· Metallic bond - a bond between atoms and ions of metals in a crystal lattice, carried out by shared valence electrons.
Thanks to this type of chemical bond, metals have a certain set of physical and chemical properties that distinguish them from non-metals.
 Rice. 3. Diagram of the crystal lattice of metals.
Rice. 3. Diagram of the crystal lattice of metals.
The strength of the metal bond ensures the stability of the crystal lattice and the plasticity of metals (the ability to undergo various processing without destruction). The free movement of valence electrons allows metals to conduct electricity and heat well. The ability to reflect light waves (ᴛ.ᴇ. metallic luster) is also explained by the structure of the metal’s crystal lattice.
However, the most characteristic physical properties of metals based on the presence of a metallic bond are:
■crystal structure;
■metallic luster and opacity;
■plasticity, malleability, fusibility;
■high electrical and thermal conductivity; and a tendency to form alloys.
Metal bond - concept and types. Classification and features of the category "Metal connection" 2017, 2018.
The very name “metallic bond” indicates that we are talking about the internal structure of metals. The atoms of most metals at the outer energy level contain a small number of valence electrons compared to the total number of outer ones that are energetically close... .
The metallic bond is based on the sharing of valence electrons belonging not to two, but to almost all metal atoms in the crystal. In metals, there are much fewer valence electrons than free orbitals. This creates conditions for free movement... .
Essential information regarding the nature of chemical bonds in metals can be obtained on the basis of two characteristic features in comparison with covalent and ionic compounds. Metals, firstly, differ from other substances in their high electrical conductivity and... .
Significant information about the nature of chemical bonds in metals can be obtained on the basis of two characteristic features of them in comparison with covalent and ionic compounds. Metals, firstly, differ from other substances in their high electrical conductivity and... .
Hybridization of orbitals and spatial configuration of molecules Type of molecule Initial orbitals of atom A Type of hybridization Number of hybrid orbitals of atom A Spatial configuration of the molecule AB2 AB3 AB4 s + p s + p + p s + p + p + p sp sp2 sp3 ... .
A metallic bond is a chemical bond caused by the presence of relatively free electrons. Characteristic of both pure metals and their alloys and intermetallic compounds. Mechanism of metallic bonding At all nodes of the crystal lattice there are... .
A molecule is the smallest particle of a substance that has its chemical properties. According to the theory of chemical bonding, the stable state of an element corresponds to a structure with the electronic formula of the outer level s2p6 (argon, krypton, radon, and others). During education... .
Topics of the Unified State Examination codifier: Covalent chemical bond, its varieties and mechanisms of formation. Characteristics of covalent bonds (polarity and bond energy). Ionic bond. Metal connection. Hydrogen bond
Intramolecular chemical bonds
First, let's look at the bonds that arise between particles within molecules. Such connections are called intramolecular.
Chemical bond between atoms of chemical elements has an electrostatic nature and is formed due to interaction of external (valence) electrons, in more or less degree held by positively charged nuclei bonded atoms.
The key concept here is ELECTRONEGATIVITY. It is this that determines the type of chemical bond between atoms and the properties of this bond.
is the ability of an atom to attract (hold) external(valence) electrons. Electronegativity is determined by the degree of attraction of outer electrons to the nucleus and depends primarily on the radius of the atom and the charge of the nucleus.
Electronegativity is difficult to determine unambiguously. L. Pauling compiled a table of relative electronegativities (based on the bond energies of diatomic molecules). The most electronegative element is fluorine with meaning 4 .
It is important to note that in different sources you can find different scales and tables of electronegativity values. This should not be alarmed, since the formation of a chemical bond plays a role atoms, and it is approximately the same in any system.
If one of the atoms in the A:B chemical bond attracts electrons more strongly, then the electron pair moves towards it. The more electronegativity difference atoms, the more the electron pair shifts.
If the electronegativities of interacting atoms are equal or approximately equal: EO(A)≈EO(B), then the common electron pair does not shift to any of the atoms: A: B. This connection is called covalent nonpolar.
If the electronegativities of the interacting atoms differ, but not greatly (the difference in electronegativity is approximately from 0.4 to 2: 0,4<ΔЭО<2 ), then the electron pair is displaced to one of the atoms. This connection is called covalent polar .
If the electronegativities of interacting atoms differ significantly (the difference in electronegativity is greater than 2: ΔEO>2), then one of the electrons is almost completely transferred to another atom, with the formation ions. This connection is called ionic.
Basic types of chemical bonds − covalent, ionic And metal communications. Let's take a closer look at them.
Covalent chemical bond
Covalent bond – it's a chemical bond , formed due to formation of a common electron pair A:B . Moreover, two atoms overlap atomic orbitals. A covalent bond is formed by the interaction of atoms with a small difference in electronegativity (usually between two non-metals) or atoms of one element.
Basic properties of covalent bonds
- focus,
- saturability,
- polarity,
- polarizability.
These bonding properties influence the chemical and physical properties of substances.
Communication direction characterizes the chemical structure and form of substances. The angles between two bonds are called bond angles. For example, in a water molecule the bond angle H-O-H is 104.45 o, therefore the water molecule is polar, and in a methane molecule the bond angle H-C-H is 108 o 28′.

Saturability is the ability of atoms to form a limited number of covalent chemical bonds. The number of bonds that an atom can form is called.
Polarity bonding occurs due to the uneven distribution of electron density between two atoms with different electronegativity. Covalent bonds are divided into polar and nonpolar.
Polarizability connections are the ability of bond electrons to shift under the influence of an external electric field(in particular, the electric field of another particle). Polarizability depends on electron mobility. The further the electron is from the nucleus, the more mobile it is, and accordingly the molecule is more polarizable.

Covalent nonpolar chemical bond
There are 2 types of covalent bonding – POLAR And NON-POLAR .
Example . Let's consider the structure of the hydrogen molecule H2. Each hydrogen atom in its outer energy level carries 1 unpaired electron. To display an atom, we use the Lewis structure - this is a diagram of the structure of the outer energy level of an atom, when electrons are indicated by dots. Lewis point structure models are quite helpful when working with elements of the second period.
H. + . H = H:H
Thus, a hydrogen molecule has one shared electron pair and one H–H chemical bond. This electron pair does not shift to any of the hydrogen atoms, because Hydrogen atoms have the same electronegativity. This connection is called covalent nonpolar .

Covalent nonpolar (symmetric) bond is a covalent bond formed by atoms with equal electronegativity (usually the same nonmetals) and, therefore, with a uniform distribution of electron density between the nuclei of atoms.
The dipole moment of non-polar bonds is 0.
Examples: H 2 (H-H), O 2 (O=O), S 8.
Covalent polar chemical bond
Covalent polar bond is a covalent bond that occurs between atoms with different electronegativity (usually, various non-metals) and is characterized displacement shared electron pair to a more electronegative atom (polarization).
The electron density is shifted to the more electronegative atom - therefore, a partial negative charge (δ-) appears on it, and a partial positive charge (δ+, delta +) appears on the less electronegative atom.
The greater the difference in electronegativity of atoms, the higher polarity connections and more dipole moment . Additional attractive forces act between neighboring molecules and charges of opposite sign, which increases strength communications.
Bond polarity affects the physical and chemical properties of compounds. The reaction mechanisms and even the reactivity of neighboring bonds depend on the polarity of the bond. The polarity of the connection often determines molecule polarity and thus directly affects such physical properties as boiling point and melting point, solubility in polar solvents.
Examples: HCl, CO 2, NH 3.
Mechanisms of covalent bond formation
Covalent chemical bonds can occur by 2 mechanisms:
1. Exchange mechanism the formation of a covalent chemical bond is when each particle provides one unpaired electron to form a common electron pair:
A . + . B= A:B
2. Covalent bond formation is a mechanism in which one of the particles provides a lone pair of electrons, and the other particle provides a vacant orbital for this electron pair:
A: + B= A:B

In this case, one of the atoms provides a lone pair of electrons ( donor), and the other atom provides a vacant orbital for that pair ( acceptor). As a result of the formation of both bonds, the energy of the electrons decreases, i.e. this is beneficial for the atoms.
A covalent bond formed by a donor-acceptor mechanism is not different in properties from other covalent bonds formed by the exchange mechanism. The formation of a covalent bond by the donor-acceptor mechanism is typical for atoms either with a large number of electrons at the external energy level (electron donors), or, conversely, with a very small number of electrons (electron acceptors). The valence capabilities of atoms are discussed in more detail in the corresponding section.
A covalent bond is formed by a donor-acceptor mechanism:
- in a molecule carbon monoxide CO(the bond in the molecule is triple, 2 bonds are formed by the exchange mechanism, one by the donor-acceptor mechanism): C≡O;
- V ammonium ion NH 4 +, in ions organic amines, for example, in the methylammonium ion CH 3 -NH 2 + ;
- V complex compounds, a chemical bond between the central atom and ligand groups, for example, in sodium tetrahydroxoaluminate Na bond between aluminum and hydroxide ions;
- V nitric acid and its salts- nitrates: HNO 3, NaNO 3, in some other nitrogen compounds;

- in a molecule ozone O3.
Basic characteristics of covalent bonds
Covalent bonds typically form between nonmetal atoms. The main characteristics of a covalent bond are length, energy, multiplicity and directionality.
Multiplicity of chemical bond
Multiplicity of chemical bond - This number of shared electron pairs between two atoms in a compound. The multiplicity of a bond can be determined quite easily from the values of the atoms that form the molecule.
For example , in the hydrogen molecule H 2 the bond multiplicity is 1, because Each hydrogen has only 1 unpaired electron in its outer energy level, hence one shared electron pair is formed.
In the O 2 oxygen molecule, the bond multiplicity is 2, because Each atom at the outer energy level has 2 unpaired electrons: O=O.
In the nitrogen molecule N2, the bond multiplicity is 3, because between each atom there are 3 unpaired electrons at the outer energy level, and the atoms form 3 common electron pairs N≡N.
Covalent bond length
Chemical bond length
is the distance between the centers of the nuclei of the atoms forming the bond. It is determined by experimental physical methods. The bond length can be estimated approximately using the additivity rule, according to which the bond length in the AB molecule is approximately equal to half the sum of the bond lengths in molecules A 2 and B 2: 
The length of a chemical bond can be roughly estimated by atomic radii forming a bond, or by communication multiplicity, if the radii of the atoms are not very different.
As the radii of the atoms forming a bond increase, the bond length will increase.
For example
As the multiplicity of bonds between atoms increases (the atomic radii of which do not differ or differ only slightly), the bond length will decrease.
For example . In the series: C–C, C=C, C≡C, the bond length decreases.
Communication energy
A measure of the strength of a chemical bond is the bond energy. Communication energy determined by the energy required to break a bond and remove the atoms forming that bond to an infinitely large distance from each other.
A covalent bond is very durable. Its energy ranges from several tens to several hundred kJ/mol. The higher the bond energy, the greater the bond strength, and vice versa.
The strength of a chemical bond depends on the bond length, bond polarity, and bond multiplicity. The longer a chemical bond, the easier it is to break, and the lower the bond energy, the lower its strength. The shorter the chemical bond, the stronger it is, and the greater the bond energy.
For example, in the series of compounds HF, HCl, HBr from left to right, the strength of the chemical bond decreases, because The connection length increases.
Ionic chemical bond

Ionic bond is a chemical bond based on electrostatic attraction of ions.
Ions are formed in the process of accepting or donating electrons by atoms. For example, atoms of all metals weakly hold electrons from the outer energy level. Therefore, metal atoms are characterized by restorative properties- ability to donate electrons.

Example. The sodium atom contains 1 electron at energy level 3. By easily giving it up, the sodium atom forms the much more stable Na + ion, with the electron configuration of the noble gas neon Ne. The sodium ion contains 11 protons and only 10 electrons, so the total charge of the ion is -10+11 = +1:
+11Na) 2 ) 8 ) 1 - 1e = +11 Na +) 2 ) 8
Example. A chlorine atom in its outer energy level contains 7 electrons. To acquire the configuration of a stable inert argon atom Ar, chlorine needs to gain 1 electron. After adding an electron, a stable chlorine ion is formed, consisting of electrons. The total charge of the ion is -1:
+17Cl) 2 ) 8 ) 7 + 1e = +17 Cl — ) 2 ) 8 ) 8
Note:
- The properties of ions are different from the properties of atoms!
- Stable ions can form not only atoms, but also groups of atoms. For example: ammonium ion NH 4 +, sulfate ion SO 4 2-, etc. Chemical bonds formed by such ions are also considered ionic;
- Ionic bonds are usually formed between each other metals And nonmetals(non-metal groups);
The resulting ions are attracted due to electrical attraction: Na + Cl -, Na 2 + SO 4 2-.
Let us visually summarize difference between covalent and ionic bond types:


Metal connection is a connection that is formed relatively free electrons between metal ions, forming a crystal lattice.
Metal atoms are usually located on the outer energy level one to three electrons. The radii of metal atoms, as a rule, are large - therefore, metal atoms, unlike non-metals, give up their outer electrons quite easily, i.e. are strong reducing agents.
By donating electrons, metal atoms turn into positively charged ions . The detached electrons are relatively free are moving between positively charged metal ions. Between these particles a connection arises, because shared electrons hold metal cations arranged in layers together , thus creating a fairly strong metal crystal lattice . In this case, the electrons continuously move chaotically, i.e. New neutral atoms and new cations constantly appear.

Intermolecular interactions
Separately, it is worth considering the interactions that arise between individual molecules in a substance - intermolecular interactions . Intermolecular interactions are a type of interaction between neutral atoms in which no new covalent bonds appear. The forces of interaction between molecules were discovered by Van der Waals in 1869, and named after him Van dar Waals forces. Van der Waals forces are divided into orientation, induction And dispersive . The energy of intermolecular interactions is much less than the energy of chemical bonds.
Orientation forces of attraction occur between polar molecules (dipole-dipole interaction). These forces occur between polar molecules. Inductive interactions is the interaction between a polar molecule and a non-polar one. A nonpolar molecule is polarized due to the action of a polar one, which generates additional electrostatic attraction.
A special type of intermolecular interaction is hydrogen bonds. - these are intermolecular (or intramolecular) chemical bonds that arise between molecules that have highly polar covalent bonds - H-F, H-O or H-N. If there are such bonds in a molecule, then between the molecules there will be additional attractive forces .
Education mechanism hydrogen bonding is partly electrostatic and partly donor-acceptor. In this case, the electron pair donor is an atom of a strongly electronegative element (F, O, N), and the acceptor is the hydrogen atoms connected to these atoms. Hydrogen bonds are characterized by focus in space and saturation
Hydrogen bonds can be indicated by dots: H ··· O. The greater the electronegativity of the atom connected to hydrogen, and the smaller its size, the stronger the hydrogen bond. It is typical primarily for connections fluorine with hydrogen , as well as to oxygen and hydrogen , less nitrogen with hydrogen .

Hydrogen bonds occur between the following substances:
— hydrogen fluoride HF(gas, solution of hydrogen fluoride in water - hydrofluoric acid), water H 2 O (steam, ice, liquid water):
— solution of ammonia and organic amines- between ammonia and water molecules;
— organic compounds in which O-H or N-H bonds: alcohols, carboxylic acids, amines, amino acids, phenols, aniline and its derivatives, proteins, solutions of carbohydrates - monosaccharides and disaccharides.
Hydrogen bonding affects the physical and chemical properties of substances. Thus, additional attraction between molecules makes it difficult for substances to boil. Substances with hydrogen bonds exhibit an abnormal increase in boiling point.
For example As a rule, with increasing molecular weight, an increase in the boiling point of substances is observed. However, in a number of substances H 2 O-H 2 S-H 2 Se-H 2 Te we do not observe a linear change in boiling points.

Namely, at water boiling point is abnormally high - no less than -61 o C, as the straight line shows us, but much more, +100 o C. This anomaly is explained by the presence of hydrogen bonds between water molecules. Therefore, under normal conditions (0-20 o C) water is liquid by phase state.
Metal connection. Properties of metallic bond.
A metallic bond is a chemical bond caused by the presence of relatively free electrons. Characteristic of both pure metals and their alloys and intermetallic compounds.
Metal link mechanism
Positive metal ions are located at all nodes of the crystal lattice. Between them, valence electrons move randomly, like gas molecules, detached from the atoms during the formation of ions. These electrons act as cement, holding the positive ions together; otherwise, the lattice would disintegrate under the influence of repulsive forces between the ions. At the same time, electrons are held by ions within the crystal lattice and cannot leave it. The coupling forces are not localized or directed. For this reason, in most cases high coordination numbers appear (for example, 12 or 8). When two metal atoms come close together, the orbitals in their outer shells overlap to form molecular orbitals. If a third atom approaches, its orbital overlaps with the orbitals of the first two atoms, resulting in another molecular orbital. When there are many atoms, a huge number of three-dimensional molecular orbitals arise, extending in all directions. Due to multiple overlapping orbitals, the valence electrons of each atom are influenced by many atoms.
Characteristic crystal lattices
Most metals form one of the following highly symmetrical lattices with close packing of atoms: body-centered cubic, face-centered cubic, and hexagonal.
In a body-centered cubic (bcc) lattice, the atoms are located at the vertices of the cube and one atom is at the center of the cube volume. Metals have a cubic body-centered lattice: Pb, K, Na, Li, β-Ti, β-Zr, Ta, W, V, α-Fe, Cr, Nb, Ba, etc.
In a face-centered cubic (fcc) lattice, the atoms are located at the vertices of the cube and at the center of each face. Metals of this type have a lattice: α-Ca, Ce, α-Sr, Pb, Ni, Ag, Au, Pd, Pt, Rh, γ-Fe, Cu, α-Co, etc.
In a hexagonal lattice, the atoms are located at the vertices and center of the hexagonal bases of the prism, and three atoms are located in the middle plane of the prism. Metals have this packing of atoms: Mg, α-Ti, Cd, Re, Os, Ru, Zn, β-Co, Be, β-Ca, etc.
Other properties
Freely moving electrons cause high electrical and thermal conductivity. Substances that have a metallic bond often combine strength with plasticity, since when atoms are displaced relative to each other, the bonds do not break. Another important property is metallic aromaticity.
Metals conduct heat and electricity well, they are strong enough, and can be deformed without destruction. Some metals are malleable (they can be forged), some are malleable (you can draw wire from them). These unique properties are explained by a special type of chemical bond that connects metal atoms to each other - a metallic bond.
Metals in the solid state exist in the form of crystals of positive ions, as if “floating” in a sea of electrons freely moving between them.
Metallic bond explains the properties of metals, in particular their strength. Under the influence of a deforming force, a metal lattice can change its shape without cracking, unlike ionic crystals.
The high thermal conductivity of metals is explained by the fact that if a piece of metal is heated on one side, the kinetic energy of the electrons will increase. This increase in energy will spread in the “electron sea” throughout the sample at high speed.
The electrical conductivity of metals also becomes clear. If a potential difference is applied to the ends of a metal sample, the cloud of delocalized electrons will shift in the direction of the positive potential: this flow of electrons moving in one direction represents the familiar electric current.
Metal connection. Properties of metallic bond. - concept and types. Classification and features of the category "Metallic bond. Properties of metallic bond." 2017, 2018.